Focusing on the future growth of Vivera’s various divisions, the Company has filed several patent applications for new medical devices, technologies, and pharmaceutical treatments utilizing the TABMELT platform.
Behind the development of each product are years of expertise, research, design, and development focused on bringing the best solutions to the market for patients and their providers.
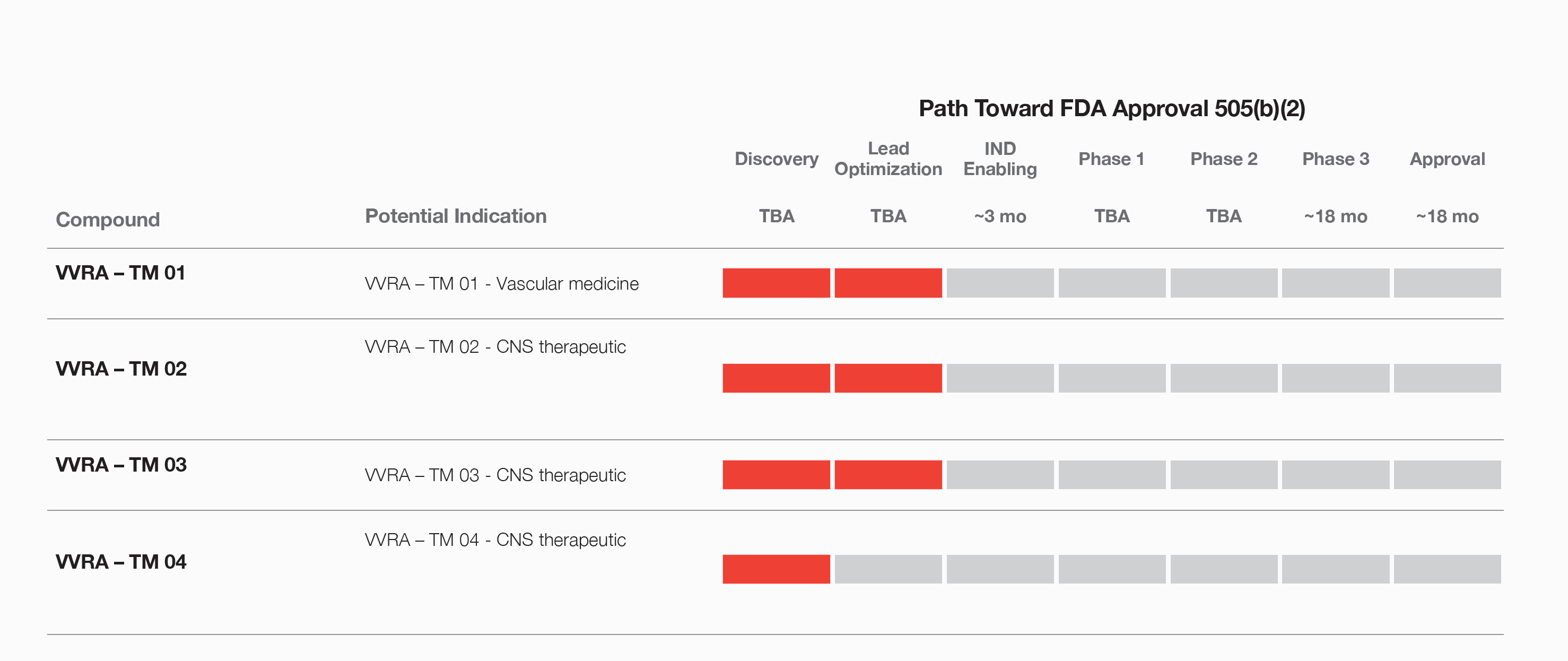
Vivera has begun laying the groundwork for clinical research trials for a variety of indications, via the Investigational New Drug (IND) and/or New Drug Application (NDA) pathways, to gain FDA approval and globally commercialize its finished pharmaceutical products.
TABMELT BioSciences Patents
Cardiovascular
| Jurisdiction | Compound | Patent Status |
| United States | VVRA – TM 001 | Published; March 2022 |
Central Nervous System
| Jurisdiction | Compound | Patent Status |
| United States | VVRA – TM 002 | Published; December 2021 |
| United States | VVRA – TM 003 | Published; March 2021 |
| United States | VVRA – TM 005 | Published; December 2021 |
| United States | PTNR – TM 006 | Published; April 2022 |
| United States | PTNR – TM 007 | Published; April 2022 |
Childhood-Onset Fluency Disorder (Stuttering)
| Jurisdiction | Compound | Patent Status |
| United States | VVRA – TM 008 | Published; January 2022 |
| United States | VVRA – TM 009 | Published; April 2023 |
Oncology
| Jurisdiction | Compound | Patent Status |
| United States | VVRA – TM 004 | Published; June 2022 |
Licensed TABMELT Patents
| Jurisdiction | Title | Patent Status |
| Australia | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Granted; August 2015 |
| United Kingdom | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Granted; May 2018 |
| Canada | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Granted; July 2018 |
| Russia | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Granted; October 2020 |
| Israel | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Granted; February 2022 |
| United States | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Published; June 2019 |
| World Intellectual Property Organization | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Published, October 2015 |
| Japan | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Filed |
| Mexico | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Filed |
| China | All Natural, Non-Toxic Sublingual Drug Delivery Systems | Filed |
ZICOH Medical Technologies Patents
| Jurisdiction | Title | Patent Status |
| United States | Smart Inhaler Device With Automated Dose Delivery, Measurement, and Management | Granted; August 2021 |
| United States | Secure Smart Dosing System With Automated Delivery, Measurement, and Management | Granted; August 2021 |
| United States | Secure Smart Dosing System With Automated Delivery, Measurement, and Management for Pills | Granted; September 2022 |
| Canada | Secure Smart Dosing System With Automated Delivery, Measurement, and Management | Published; February 2020 |
| Canada | Secure Smart Dosing System With Automated Delivery, Measurement, and Management | Published; March 2021 |
| United Kingdom | Smart Inhaler Device With Automated Dose Delivery, Measurement, and Management | Granted; August 2021 |
| Australia | Smart Inhaler Device With Automated Dose Delivery, Measurement, and Management | Published; March 2021 |
| Israel | Smart Inhaler Device With Automated Dose Delivery, Measurement, and Management | Published; May 2022 |
| World Intellectual Property Organization | Smart Inhaler Device With Automated Dose Delivery, Measurement, and Management | Published; February 2020 |
| World Intellectual Property Organization | Smart Inhaler Device With Automated Dose Delivery, Measurement, and Management | Published; March 2021 |
Other Vivera Technologies Patents
| Jurisdiction | Title | Patent Status |
| United States | Self-Contained, Portable Sanitation Device and Telemedicine Station | Granted; August 2022 |
| United States | Self-Contained, Portable Sanitation Device and Telemedicine Station | Published; April 2023 |
| United States | Fluency Evaluation and Feedback | Granted; February 2023 |
| United States | Self-Administered Infection Testing and Result Determination | Published; October 2021 |